The manufacturer of eye drops that have been linked to an outbreak of serious bacterial infections in the US, including at least three deaths, did not follow proper protocol to prevent contamination of its products, according to the FDA.
The manufacturer of eye drops that have been linked to an outbreak of serious bacterial infections in the US, including at least three deaths, did not follow proper protocol to prevent contamination of its products, according to an inspection report published Friday by the US Food and Drug Administration.
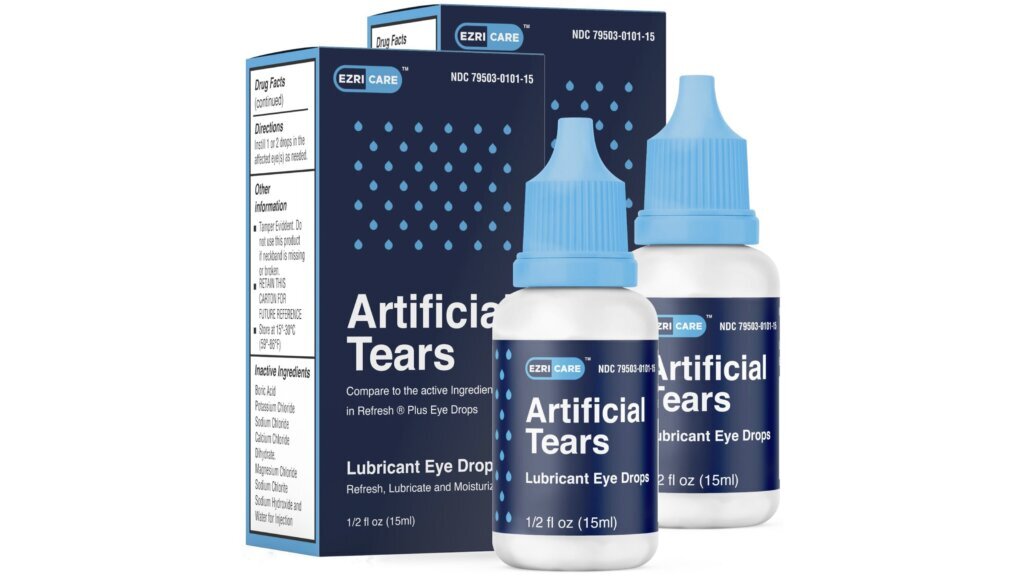
The FDA visited a Global Pharma Healthcare facility in India for an inspection that started in mid-February, 2½ weeks after the company recalled EzriCare Artificial Tears due to possible contamination.
At the time of the recall, there were 55 reports of adverse events including eye infections, permanent loss of vision and at least one death with a bloodstream infection. As of late last month, 68 infections had been identified in 16 states, according to the US Centers for Disease Control and Prevention. There have been three deaths, eight cases of vision loss and four surgical eye removals reported.
An 11-day inspection of the Global Pharma facility resulted in 11 observations by the FDA, including a “manufacturing process that lacked assurance of product sterility,” specifically for batches of product that were manufactured between December 2020 and April 2022 and shipped to the US.
The EzriCare Artificial Tears product, which is manufactured by Global Pharma, is part of an outbreak of infections from bacteria called Pseudomonas aeruginosa.
This rare drug-resistant bacteria can spread among people who don’t have symptoms — and to people who haven’t used the eye drops, according to the CDC. This type of spread is particularly common in health care settings.
“The bacteria can spread when one patient carrying the bacteria exposes another patient, or when patients touch common items or when healthcare workers transmit the germs which is why infection control, like hand hygiene, is so important,” the agency told CNN in an email Monday.
Several cases in the current outbreak have been identified in people who were carrying the bacteria without signs or symptoms of clinical infections, the CDC said. These cases were discovered through screenings at inpatient health care facilities that had clusters of infections.
The particular strain of the bacteria associated with this outbreak had never before been reported in the US, and related infections have been identified at acute care hospitals, long-term care facilities, emergency departments, urgent care clinics and other outpatient facilities.
People affected by the outbreak reported using different brands of artificial tears, but EzriCare Artificial Tears was most commonly reported.
The FDA inspection of the Global Pharma facility is part of an ongoing compliance matter.
“The FDA’s highest priority is protecting public health — this includes working with manufacturers to quickly remove unsafe drugs from shelves when they are identified,” the agency said in an email Monday. “The FDA continues to monitor this issue and is working with the Centers for Disease Control and Prevention (CDC) and the companies recalling these affected products. We urge consumers to stop using these products which may be harmful to their health.”
The-CNN-Wire
™ & © 2023 Cable News Network, Inc., a Warner Bros. Discovery Company. All rights reserved.
Stay connected with us on social media platform for instant update click here to join our Twitter, & Facebook
We are now on Telegram. Click here to join our channel (@TechiUpdate) and stay updated with the latest Technology headlines.
For all the latest Health & Fitness News Click Here
